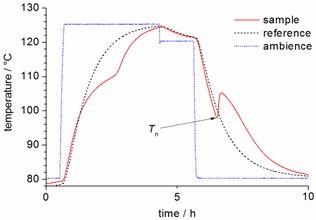
Nucleation
|
Many solid-liquid PCM exhibit supercooling, i.e. the crystallisation of the PCM upon cooling starts at a lower temperature than the melting temperature. The temperature at the starting point for crystallisation is called nucleation temperature. Nucleation theory of the liquid-solid phase change describes the formation of a solid particle in the bulk of the liquid phase. This process is spontaneous under isobaric and isothermal conditions if it is associated with a reduction in the Gibbs’ potential, which applies for temperatures below the equilibrium melting temperature. In the course of the phase change, the liquid and the solid phase meet at an interface which is associated with an interface energy. The formation of the interface, however, is in any case an endothermic process associated with the surface tension. Thus, at a temperature below the equilibrium melting temperature, the net change in Gibbs’ potential is negative only beyond a certain cluster size, the so-called critical radius. Only clusters larger than the critical radius will grow spontaneously and the whole material will solidify consequently. This nucleation barrier can be overcome by thermal fluctuations. Since these fluctuations are local stochastic events, nucleation is a probabilistic phenomenon. Thermal fluctuations as well as the nucleation barrier depend on temperature, and therefore the nucleation rate is also dependent on temperature. Two types of nucleation can be distinguished: homogeneous and heterogeneous nucleation. In the case of homogeneous nucleation, only the phase changing substance is involved and the surface tension refers to the interface between solid and liquid. Homogeneous nucleation can take place at any location in the system, and the whole volume is a potential nucleation site. Hence the nucleation rate is a function of the bulk volume. However, if a suitable foreign substance is present providing a smaller surface tension with respect to the solid, nucleation will occur preferably at the interface to this foreign substance (heterogeneous nucleation). Substances with a particularly small surface tension for a given solid (nucleating agents) can be added to enhance nucleation. In principle any impurity or surface in contact with the liquid has the potential to offer a reduced surface tension and to overcome the nucleation barrier. Summarising, the observed nucleation temperature depends on the volume and the purity of the sample under investigation. The larger the volume and the more impurities are present, the higher the absolute probability for a nucleation event is and the closer the observed nucleation temperature is expectably to the melting temperature. In addition, the nucleation temperature depends on the applied cooling rate: the lower the applied cooling rate, the closer the observed nucleation temperature is expectably to the melting temperature. Literature:
|
Nucleation temperature T_n of a PCM sample cooled down exposed to a constant ambient temperature |
|---|